The eliciting dose of peanut in double-blind, placebo-controlled food challenges decreases with increasing age and specific IgE level in children and young adults
Tjitske van der Zee , MD Anthony Dubois , MD, PhD , Marjan Kerkhof , MD, PhD , Sicco van der Heide , PhD , Berber Vlieg-Boerstra , PhD, RD
Background
Several risk factors for severe anaphylactic reactions to food in daily life are known. However, to date, it is not possible to predict the severity of allergic reactions to food in the individual patient with accuracy. Some studies show that a history of severe reactions is associated with a lower eliciting dose in double-blind, placebo-controlled food challenges (DBPCFCs). Therefore, in this study, the eliciting dose was used as a measure of clinical sensitivity.
Objectives
To study whether risk factors for severe allergic reactions to food in daily life such as age, degree of sensitization, and coexistent atopic disease influence the eliciting dose in DBPCFCs in children allergic to peanut.
Methods
Data from children who had clinical reactions to peanut during DBPCFCs at the University Medical Center Groningen (2001-2009) were analyzed. A Cox regression model was used to analyze the association of the determinants with the eliciting dose.
Results
One hundred twenty-six positive DBPCFCs with peanut were analyzed. Age older than 10 years, a specific IgE level above the lowest tertile (≥5.6 kU/L), and the absence of atopic dermatitis were associated with reactions to lower doses: respective hazard ratios 1.89 (95% CI, 1.28-2.81; P = .001), 2.03 (95% CI, 1.37-3.00; P < .0001), and 0.45 (95% CI, 0.29-0.71; P = .001) present versus absent. No significant associations with the eliciting dose were found for sex, the presence of asthma and rhinitis, and the severity of food reactions by history.
Conclusions
Using the eliciting dose as a measure of clinical sensitivity, greater clinical sensitivity in DBPCFCs to peanut was found to be associated with increasing age, higher specific IgE level, and the absence of atopic dermatitis. This finding may explain why adolescents experience severe allergic reactions in daily life to peanut more often than do younger children.
Link to JACI article: http://www.jacionline.org/article/S0091-6749(11)01239-5/fulltext
Download PDF with tables and images: http://www.jacionline.org/article/S0091-6749(11)01239-5/pdf
LAYPERSON EXPLANATION, By Dr Eli Silver
A food allergy research group from Netherlands was trying to zero-in on the risk factors for severe peanut reactions.
They have discovered that an older age, in itself, is a risk factor for more a severe peanut allergy.
In the table below, one can see that it takes a lesser amount of peanut to provoke an allergic reaction among older patients, > 10 y.o. as compared to that among preschoolers, <5 y.o. In other words, it took 10 x fold more peanut protein to cause a reaction in a preschooler as compared to a teenager.
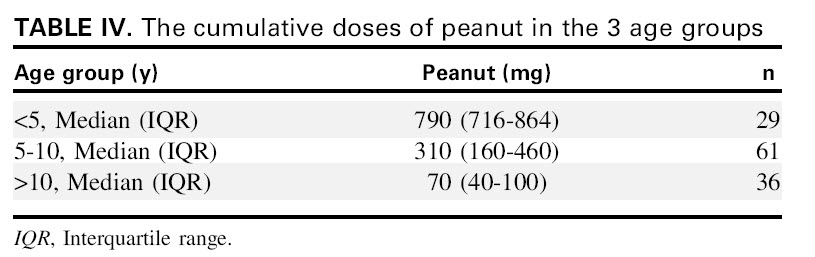
TABLE IV. as you seen, it takes 790 mg of peanut to provoke a reaction in child under 5 y.o as opposed to only 70 mg of peanut to start a reaction in a child older than 10 years.
Also, a higher peanut IgE antibody level was associated with a more severe reaction: “For each increase of 1 kU/L in the peanut-specific antibody IgE level, the risk of reacting to a specific eliciting dose in increased by 1%”, the authors wrote.
In other words, the “perfect storm” of peanut allergy would be a teen with a high peanut antibody level. This is terrible, because for many years we have observed that more severe and deadly peanut reactions tend to happen among teenagers! The common “wisdom” to explain this finding was that the teens are more opt for risk-taking behavior and may not keep the epinephrine handy.
Yet, this Dutch study has discovered that, at least, a part of the explanation is that the very severity of peanut allergy increases with age.
Bottom line, teens are going be teens: we can’t always change their behavior and chain them down to an Epipen, yet, we know that the oral immunotherapy can mitigate this risk and “peanut”-proof our kids. The OIT may even be safer among younger children, as their intrinsic peanut sensitivity has not yet reached its peak.
“Like any study, this report has its limitations, and I am looking forward to see more data published by the Dutch group, but in my mind, it’s a prime time for OIT, now; for peanut sensitivity “does not waste any time”. ~ Eli Silver MD