 Richard Wasserman, M.D., Ph.D.
Richard Wasserman, M.D., Ph.D.
Education:
- S., Chemistry Hobart College, Geneva, NY
- D. Biomedical Sciences, University of New York, Mt. Sinai School of Medicine, NY, NY
- D. Mt. Sinai School of Medicine / University of Texas Southwestern Medical School
- Pediatrics Residency Children’s Hospital of Philadelphia
- Fellowship, Bone Marrow Transplantation / Immunology Children’s Hospital of Philadelphia
- Fellowship, Postdoctoral Cancer / Immunology University of Texas Southwestern Medical School
- Fellowship, Immunology / Rheumatology The Rockefeller University
Professional Interests:
- Immunodeficiency, Asthma, Food Allergy
Certifications & Licensure
- American Board of Allergy and Immunology-Allergy & Immunology
- American Board of Pediatrics-Pediatrics
- TX State Medical License
Awards, Honors, & Recognition
- Achievements and Innovations Award for Best Workflows “in recognition of important accomplishments, innovations and solutions that advance effective electronic medical records.”Centricity Healthcare User Group, 2005
- Allergy/Immunology Teacher of the Year-University of Texas Southwestern Medical School, 1995, 1996
- Volunteer of the Year 1995-1996American Lung Association-North Texas Affiliate, 1995, 1996
- Founder’s Award Southern Society for Pediatric Research1988
- Sigma Xi:1983-date1983
- Recipient of the Glorney-Raisbeck Fellowship of the New York Academy of Medicine1980, 1981
- Phi Beta Kappa1970
- Top MD-Consumers Checkbook
- Fellow (FAAAAI)American Academy of Allergy Asthma and Immunology
- Super Doctor-com
Professional Memberships
- American Academy of Allergy Asthma and Immunology – AAAAI Fellow
- American College of Allergy, Asthma and Immunology Fellow
- Clinical Immunology Society Member
Hospital Affiliations
- Medical City Dallas Hospital-Dallas, TX
- Children’s Health System of Texas-Dallas, TX
- Baylor University Medical Center-Dallas, TX
Providing Hope for People with Food Allergies
Our innovative food allergy immunotherapy program has helped hundreds of children overcome a fear of food.
Dallas Food Allergy Center (DFAC) is the food allergy specialty practice at Allergy Partners of North Dallas. We are one of the few practices in the country that offer a breakthrough oral immunotherapy program for kids with food allergies. Our treatment provides a long-term solution for wheat, egg, peanut, tree nut, and milk allergic patients. It is usually takes less than 6 months, and at the end of the program, most patients with allergies to wheat, egg, peanut, tree nut, or milk are able to consume these foods with no allergic reaction.
More than 80% of our patients can now safely consume foods that once threatened their health.
The DFAC program has helped hundreds of children by setting them free from fear of their food allergies. The treatment works by introducing minute doses of wheat, egg, peanut or milk in solution for approximately 6 months (time period varies on individual differences). The program then progresses to small doses of the whole food for an additional several months, allowing the patient to eat these foods. As a result, the vast majority of our patients are able to consume wheat, eggs, peanuts and/or milk without any adverse reactions.
What would it mean to your family if you were free from worrying about severe food allergies?
Families tell us we have changed their lives significantly by helping their children eat foods that used to be harmful. The impact on family life has made a difference for not only the allergic child, but also for siblings and parents. Many children have graduated from the DFAC Food OIT program and are now eating eggs, wheat, tree nuts, or peanuts or are drinking milk without reactions.
What to expect from the DFAC food allergy program:
This program is designed for a child of school age or older who will be old enough to understand the reasons for participating and be actively engaged in the process.
The first visit of the oral immunotherapy process is a full day during which the child will receive multiple doses of very small amounts of diluted milk, diluted wheat powder, diluted peanut (or nut) powder, or diluted egg powder. The child will return weekly for dose increases followed by one hour of observation. Between each visit the patient will take two doses of the desensitization (allergenic) food each day at home. Once oral immunotherapy is complete, the previously allergenic food can be freely included in the diet. The food must be eaten daily to maintain the ability to eat it safely.
Want to know about treating your child’s food allergy?
If you are interested in learning more about how your child can be treated for food allergies, please contact our office at 972-566-7788. Most insurance policies cover this treatment. We invite you to call us and find out how we can help your child as well as your whole family live without a fear of food.
Note to Allergists/Immunologists: If you would like to obtain a copy of the oral immunotherapy procedures we use, please send an email request to drrichwasserman@gmail.com
Include a link to your website or enough identifiers so that I can confirm that you are Certified by the American Board of Allergy and Immunology. After confirmation we will provide you with the information requested.
Contact
Richard Wasserman, M.D., Ph.D.
Dallas: 7777 Forest Ln #B332, Dallas, TX 75230
Frisco: 4500 Hillcrest Rd, Frisco, TX 75035
(972) 566-7788
http://dallasallergy.net
DallasAllergyImmunology / Allergy Partners of North Texas: Dr. Richard Wasserman, Dr. Robert Sugerman, Dr. Stacy Silvers, Dr. Qurat Kamili
OIT Details
State: Texas
Treats single/multi allergens at once: Both options: have done up to 4 nuts and would be open to other combinations.
Offers SLIT for food allergens: No
Additional Features:
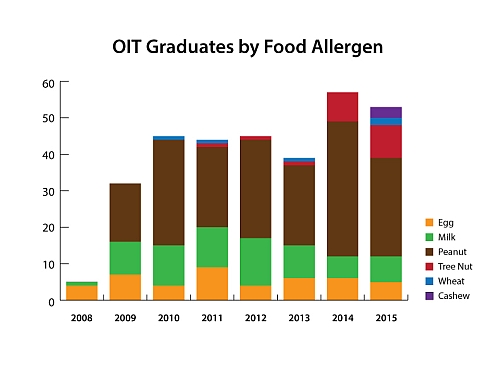
Over 300 OIT graduates!
Articles in Our Research & Learn Center
- BLOG: Just a Little Peanut; by Julie and Alexander
- BLOG: Calista's Peanut Allergy Adventures; by Becky and Calista
- Dr. Wasserman interview: OIT...Just the Facts
- Dr. Wasserman: Dallas clinic tries new strategy for dealing with kids’ allergies; The Dallas Morning News, 2015
- Calling all allergists! Dr. Wasserman, PhD and MD, will share his OIT protocol with board-certified allergists
- Dr. Wasserman: Dallas doctors fight fire with fire using experimental desensitizing therapy; Dallas Morning News, 2014
- Editorial: Oral Immunotherapy for the Treatment of Peanut Allergy: Is It Ready for Prime Time? 2014
- Oral immunotherapy for peanut allergy: multipractice experience with epinephrine-treated reactions. 2014
- Dr. Wasserman: Controversial Treatment Ends Food Allergies; NBCDFW TV, NBC affiliate; 2010
- BLOG: Fed Up: Our Food Allergy Oral Immunotherapy Journey; by Katie and Brendan
 Map to Office(s)
Map to Office(s)
[property_map1]
[property_map2]
[property_map3]
[property_map4]
[property_map5]
Publications & Presentations
PubMed
- Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency.Wasserman, R. L., Melamed, I., Stein, M. R., Gupta, S., Puck, J., Engl, W., Leibl, H., McCoy, B., Empson, V. G., Gelmont, D., Schiff, R. I.; J Allergy Clin Immunol. 2012 Oct.
- Hereditary angioedema:Validation of the end point time to onset of relief by correlation with symptom intensity.Bernstein J Ritchie B Levy R Wasserman R Bewtra A Hurewitz D Obtulowicz K Reshef A Moldovan D Shirov T Grivcheva-Panovska V Kiessling P Keinecke HO Craig T ; Allergy Asthma Proc . 2010 Oct 19 .
- Recurrent Granulibacter bethesdensis infections and chronic granulomatous disease.Greenberg DE Shoffner AR Zelazny AM Fenster ME Zarember KA Stock F Ding L Marshall-Batty KR Wasserman RL Welch DF Kanakabandi K Sturdevant DE Virtaneva K Porcella SF M…; Emerg Infect Dis . 2010 Sep .
Journal Articles
- Efficacy of Human C1-Esterase Inhibitor (C1-INH) concentrate compared to placebo in acute abdominal hereditary angioedema attacks. Craig TJ, Levy RJ, Wasserman RL, et al, J Allerg Clin Immunol, 104(4), 801-808
- Use of intravenous immunoglobulin and adjunctive therapies in the treatment of primary immunodeficiencies. Yong, Pierre L & Boyle, John & Ballow, Mark & Boyle, Marcia & Berger, Melvin & Bleesing, Jack & Bonilla, Franciso A & Chinen, Javier & Cunninghamm-Rundles, Charlotte &…, Clin Immunol. 135(2):255-63
- 2010 Prospective study of rapid relief provided by C1 esterase inhibitor in emergency treatment of acute laryngeal attacks in hereditary angioedema. Craig TJ, Wasserman RL, Levy RJ, Bewtra AK, Schneider L, Packer F, Yang W, Keinecke HO, Kiessling PC, J Clin Immunol. 30:823–829
- Pharmacokinetics and Safety of Subcutaneous Immune Globulin (Human), 10% Caprylate/Chromatography Purified in Patients With Primary Immunodeficiency Disease. R L Wasserman, Irani AM, Tracy J, Tsoukas C, Stark D, Levy R, Chen J, Sorrells S, Roberts R, Gupta S, Clin Exp Immunol. 161(3), 518–526
- Orange JS 2010 Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with primary immunodeficiency. JB Hagan, Fasano MB, Spector S, Wasserman RL, Melamed I, Rojavin MA, Zenker O, J Clin Immunol. 30(5):734-45
- Population Pharmacokinetics of Plasma-Derived C1 Esterase Inhibitor Concentrate Used to Treat Acute Hereditary Angioedema Attacks. Bernstein JA, Ritchie B, Levy RJ, Wasserman RL, Bewtra AK, Hurewitz DS, Obtulowicz K, Reshef A, Moldovan D, Shirov T, Grivcheva-Panovska V, Kiessling PC, Schindel F, C…, Ann Allergy Asthma Immunol. 105(2):149-54
- Prospective study of C1 esterase inhibitor in the treatment of successive acute abdominal and facial hereditary angioedema attacks. RL Wasserman, Levy RJ, Bewtra AK, Hurewitz D, Craig TJ, Kiessling PC, Keinecke HO,Bernstein JA, Ann Allergy Asthma Immunol. 106(1): 62-68
- Pharmacokinetics of Subcutaneous IgPro20 in patients with primary immunodeficiency. Wasserman RL, Melamed I, Nelson RP, Knutsen AP, Fasano MB, Stein MR, Rojavin MA, Kiessling P, Church JA, Clin Pharmacokinet. 50(6): 405-414
- Efficacy, Safety, and Pharmacokinetics of a 10% Liquid Immune Globulin Preparation (GAMMAGARD LIQUID, 10%) Administered Subcutaneously in Subjects with Primary Immunod… Wasserman RL, Melamed I, Kobrynski L, Strausbaugh SD, Stein MR, Sharkhawy M, Engl W, Leibl H, Sobolevsky L, Gelmont D, Schiff RI, Grossman WJ, J Clin Immunol. 2011 Jun;31(3):323-31
- Human pasteurized C1 esterase inhibitor concentrate in 1085 hereditary angioedema attacks – final results of the open-label extension study. Craig TJ, Bewtra AK, Bahna SL, Hurewitz D, Schneider L, Levy R, Moy J, Offenberger J, Jacobson KW, Yang W, Eidelman F, Janss G,Packer F, Rojavin MA, Machnig T, Keineck…, I.M.P.A.C.T.2. 2011 Allergy. 2011 Sep 2. doi: 10.1111/j.1398-9995.2011.02702.x
- Safety, Efficacy And Pharmacokinetics Of A New 10% Liquid Intravenous Immunoglobulin (Bivigam™ IVIG) In Patients With Primary Immunodeficiency. Wasserman RL,Church J, Stein M, Moy J, White M, Strausbaugh S, Schroeder H, Ballow M, Harris M, Melamed I, Elkayam D, Lumry W, Suez D, Rehman SM, J Clin Immunol. 2012 Aug;32(4):663-9
- Safety of L-proline as a stabilizer for immunoglobulin products. Hagan JB, Wasserman RL, Baggish J, Spycher M, Berger M, Shashi V, Lohrmann E, Sullivan K, Expert Rev Clin Immunol 8(2) 169-187
- Evaluation of correlation between dose and clinical outcomes in subcutaneous immunoglobulin replacement therapy. Orange JS, Belohradsky BH, Berger M, Borte M, Hagan J, Jolles S, Wasserman RL, Baggish JS, Saunders R, Grimbacher B, Clin Exp Immunology. 2012: 169:172-181
- Treatment response after repeated administration of C1 esterase inhibitor for successive acute hereditary angioedema attacks. Craig TJ, Bewtra AK, Hurewitz D, Levy R, Janss G, Jacobson KW, Packer F, Bernstein JA, Rojavin MA, Machnig T, Keinecke OH, Wasserman RL, Asthma and Allergy Proc. 33:354-61
- Novel Method of Subcutaneous Immunoglobulin Administration Facilitated by Recombinant Human Hyaluronidase: A Phase Three Clinical Study of Efficacy, Tolerability and P… Wasserman RL, Melamed I, Stein MR, Engl W, Leibl H, McCoy B, Empson VG, Gelmont D, Schiff RI and the IGSC, 10% with rHuPH20 Study Group, J. Allergy Clin Immunol. 2012. 130:951-57
- C1-INH concentrate for treatment of acute hereditary angioedema: a pediatric cohort from the I.M.P.A.C.T. studies. Schneider L, Hurewitz D, Wasserman R, Obtulowicz K, Machnig T, Moldovan D, Reshef A, Craig TJ, Pediatr Allergy Immunol. 2013 Feb;24(1):54-60. doi: 10.1111/pai.12024
- Per-Attack Reporting of Prodromal Symptoms Concurrent with C1-Inhibitor Treatment of Hereditary Angioedema Attacks. Prematta M, Bewtra AK, Levy RJ, Wasserman RL, Jacobson KW, Machnig T, Craig T, Adv Ther, 1-10. DOI 10.1007/s12325-0053-5
- C1-inhibitor therapy for hereditary angioedema attacks: Prospective patient assessments of health-related quality of life. Bewtra AK, Levy RJ, Jacobson KW, Wasserman RL, Machnig T, Craig TJ, Allergy and Asthma Proc. 33:427-431
- Transfusion-associated cytomegalovirus infection and acquired immune deficiency syndrome in an infant. Shannon K, Ball E, Wasserman RL, Murphy FK, Luby J, Buchanan GR, J. Pediatr. 103:859-863
- Ataxia -Telangectasia associated with sarcoidosis. Fleck RM, Meyers LK, RL Wasserman, Tigelaar RE, Freeman RG, Pediatr. Derm. 3:339-343
- HIV infection detected by the PCR in an agammaglobulinemic patient. Young KKY, Peter JB, Wasserman RL, AIDS 4:468-469
- Cephaloskeletal dysplasia (Tabyi-Linder syndrome; osteodysplastic primordial dwarfism type III): report of two cases and a review of the literature. Vichi GF, Currarino G, Wasserman RL, Duvina PL, Filippi L, Pediatr Radiol 30:644-652
- Office-based oral immunotherapy for food allergy is safe and effective. Wasserman RL, Sugerman RW, Mireku-Akomeah N, Mansfield L, Baker JW, J Allergy Clin Immunol. 2011 Jan;127(1):290-1; author reply 291-2
- Neonatal Sepsis: The Potential of Granulocyte Transfusion. Wasserman RL, Hospital Practice 17:95-104
- Monoclonal Antibodies: Progress and Promise. Polin RA, Wasserman RL, Lab. Manage. 21:33-38,40,43,44
- Unconventional therapies for neonatal sepsis. Wasserman RL, Pediatr. Inf. Dis. 2:421-423
- Caustic Substance Injuries. Wasserman RL, Ginsburg C, J. Pediatr. 107:169-174
- Intravenous Gammaglobulin Prophylaxis for Newborn Infants. Wasserman RL, Pediatr. Inf. Dis. 5:620-621
- Perinatally acquired AIDS. Wasserman RL, Dallas Med. J. 73:292-295
- Perinatal Aids. Wasserman RL, 1993 AJCEN 1993/5 Review Issue: 40-47
- IgG subclass determinations. Wasserman RL, Pediatr. Inf. Dis. 7:303
- Antibody deficiency – ubiquitous problem or useless concept? Wasserman RL, Eur J Pediatr (1988) 147:458-459
- Immunodeficiency that is not AIDS – Primary Immunodeficiency Disease. Wasserman RL, Dallas Medical Journal
- Evaluating humoral immunity: The role of immunization with bacterial polysaccharide vaccine. Wasserman RL, Sorensen RU, Pedia Inf Dis J. 1999, 18(2):157-163
- Pediatric Asthma Management in the 21st Century. Wasserman RL, Texas Pharm. 2002, 122(4) 22-24,35
- Advances in the Treatment of Pediatric Asthma. Infectious Disease in Children. Brunell, PA, Irani, MA, Kattan, M, Martinez, FD, Shapiro, G, Wasserman, RL, CME supplement
- Tribute to Dale Coln, MD, upon his retirement. Wasserman RL, Baylor Univ. Med. Ctr. Proc. 2005, 18(4) 214
- Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthm… Orange JS, Hossney EM, Weiler CR, et. al, J Allergy Clin Immunol. 2006 Apr;117(4 Suppl):S525-53
- Subcutaneous Immunoglobulin: Opportunities and Outlook. Misbah S, Sturzenegger MH, Borte M, Shapiro RS, Wasserman RL, Berger M, Ochs HD, Clin Exp Immunol 158:s1:1-70
- Use of intravenous immunoglobulin and adjunctive therapies in the treatment of primary immunodeficiencies: A working group report of and study by the Primary Immunodef… Yong PL, Boyle J, Ballow M, Boyle M, Berger M, Bleesing J, Bonilla FA, Chinen J, Cunninghamm-Rundles C, Fuleihan R, Nelson L, Wasserman RL, Williams KC, Orange JS, Clin Immunol. May;135(2):255-63
- Diagnosis and Treatment of Primary Immunodeficiency Disease: The Role of the Otolaryngologist. Wasserman RL, Manning SC, Am J. Otolaryncol. 32(4):329-337
- The Art of IgG Treatment in Treating Primary Immunodeficiency Patients. Wasserman RL, Ballow M, Shapiro R, Stein MR, Immunol Allergy Clin NA
- Progress in Gammaglobulin Therapy for Immunodeficiency: From Subcutaneous to Intravenous Infusions and Back Again. Wasserman RL, J Clin Immunol. (DOI) 10.1007/s10875-012-9740-x. 2012. 32:1153-64
- Phylogenetically associated residues among the VHIII subgroup of several mammalian species. Capra JD, Wasserman RL, Kehoe JM, J. Exp. Med. 138:410-427
- The VHIII subgroup of immunoglobulin heavy chains: Phylogenetically associated residues in several avian species. Wasserman RL, Kehoe JM, Capra JD, J. Immunol. 113:954-957
- The aminoterminal sequence of the VHIII subgroup of pooled porcine IgG. Franek F, Wasserman RL, Novotny J, Kehoe JM, Eur. J. Immunol. 5:427-429
- Primary structure of the variable regions of two canine immunoglobulin heavy chains. Wasserman RL, Capra JD, Biochem. 16:3160-3168
- Amino acid sequence of the Fc region of a canine immunoglobulin M: Interspecies homology for the IgM class. Wasserman RL, Capra JD, Science 200:1159-1161
- The amino acid sequence of the light chain variable region of a canine myeloma immunoglobulin: Evidence that the VK subgroups predated mammalian speciation. Wasserman RL, Capra JD, Immunochem. 15:303-305
- The use of EBV-transformed B cell lines for the generation of Ig-producing human B cell hybridomas. Chiorazzi N, Wasserman RL, Kunkel HG, J. Exp. Med. 156:930-935
- In vitro stimulation prior to fusion generates antigen binding human-human hybridomas. Wasserman RL, Budens R, Thaxton ES, J. Immunol. Methods. 93:275-283
- The complete protein sequences of the variable regions of the cloned heavy and light chains of a human anti-cytomegalovirus antibody reveal a striking similarity to hu… Newkirk MM, Gram H, Heinrich GF, Ostberg L, Capra JD, Wasserman RL, J. Clin. Invest. 81:1511-1518
- Can children transmit human immunodeficiency virus infection? Rogers MF, White CR, Sanders R, Schable C, Krell TE, Wasserman RL, Beuanti JA, Peters SM, Wray BB, Pediatr. 85:210-214
- Demonstration by enzymatic RNA amplification of persistent enterovirus infection in culture-negative meningoencephalitis. Rotbart HA, Kinsella JP, Wasserman RL, J. Inf. Dis. 161:787-791
- Safety and tolerability of an intravenous immune globulin at various concentrations in 5% dextrose injection or sterile water for injection. Miller K, Wasserman RL, Clin Pharm 11:628-31
- Chronic Sinusitis as a Manifestation of Primary Immunodeficiency in Adults. Manning SC, Wasserman RL, Leach, J and Truelson, Amer J Rhinology 8:29-35
- Stable Enterovirus 5’ nontranslated region over a 7-year period in a patient with agammaglobulinemia and chronic infection. Dunn JJ, Romero JR, Wasserman R, Rotbart HA, J Inf. Disease. 182(1): 298-301
- Efficacy, Safety and tolerability of a New Intravenous Immune Globulin, 10% Liquid, Triple Virus Reduced (IGIV, 10% TVR) in Patients With Primary Immunodeficiency (PID). Church JA, Leibl H, Stein MR, Melamed IR, Rubinstein A, Schneider LC, Wasserman RL, et. al, J Clin Immunol. 26(4): 388-95
- Efficacy and safety of inhaled fluticasone propionate chlorofluorocarbon in 2- to 4-year-old patients with asthma: results of a double-blind, placebo-controlled study. Wasserman, RL, Kim KT, Blake K, et al, Ann Allrg, Asthma, Immunol. 96(6): 808-818
- Fluticasone Propionate/Salmeterol HFA via Metered Dose Inhaler with Integrated Dose Counter: Performance and Patient Satisfaction. K Sheth, Wasserman RL, Lincourt WR, et. al, Int J Clin Prac. 60(10): 1218
- Real World Assessment of a Metered- Dose Inhaler with Integrated Dose Counter. RL Wasserman, Sheth K, Lincourt WR, et. al, Allergy Asthma Proc. 27:486-92
- Intrainfusion and postinfusion adverse events related to intravenous immunoglobulin therapy in immunodeficiency states. H Tcheurekdjian, Martin J, Kobayashi R, Wasserman RL, Hostoffer R, Allergy Asthma Proc. 27:532-536
- Expert opinion regarding clinical and other outcome considerations in the formulary review of immune globulin. Sorensen R, Dinakar C, Fuleihan R, Gupta S, Knutsen A, Kobayashi R, Koski CL, Levinson A, Shapiro R, Wasserman RL, J Manag Care Pharm. 13(3):278-283
- Safety and Efficacy of Privigen®, a Novel Liquid 10% Immunoglobulin Preparation for Intravenous Use in Patients with Primary Immunodeficiencies. Stein M, Nelson RP, Church JA, Wasserman RL, et al, J Clin Immunol. 29(1):137-44
Books/Book Chapters
Pharmacokinetics of a new 10% Intravenous Immunoglobulin. Chapter: Pharmacokinetics of a new 10% Intravenous Immunoglobulin Page 272 – 278
Wasserman RL, Church JA, Peter HH, et.al
Editors: .Eur J Pharm Sci 37, 28
Structure and evolution of immunoglobulin heavy chain variable regions. Page 3 – 18
Capra JD, Wasserman RL, Querinjean P, Kehoe JM
Editors: .FEBS Proceedings, Budapest
The Glycoconjugates. Chapter: Immunoglobulins
Wasserman RL, Capra JD
Editors: MI Horowitz, W Pigman.Academic Press, New York
Biological Basis of Immunodeficiency. Chapter: Primary structural conservation in the evolution of IgM Page 169 – 176
McCumber LJ, Wasserman RL, Capra JD
Editors: EW Gelfand, HM Dosch.Raven Press, New York
Immunohematology. Chapter: Monoclonal Antibodies: Production and Use
Wasserman RL
Editors: E. Steane.American Dade, Miami
Current therapy in pediatric infectious disease. Chapter: Chronic granulomatous disease Page 275 – 279
Wasserman RL
Editors: JD Nelson.B.C. Decker Inc, Toronto
Current therapy in pediatric infectious disease. Chapter: Antibody deficiency states Page 271 – 275
Wasserman RL
Editors: JD Nelson.B.C. Decker Inc., Toronto
Human disease: Autoimmunity and human heavy chain variable regions. Chapter: Polymorphism’s of immunologically relevant loci Page 378 – 382
Sanz I, RL Wasserman, Hwang LY, Hasemann C, Thomas J, Tucker P, Capra JD
Editors: F. Morriss.Quality Medical Publishing Inc., St. Louis
Essentials Pediatric Intensive Care. Chapter: Immunologic Disorders Page 172 – 174
Wasserman RL
Editors: D Levin and F Morriss.Quality Medical Publishing, Inc., St. Louis
1001 Healthy Baby Answers. Chapter: Asthma Page 93 – 98
Wasserman RL
Editors: GC Morchower.Sourcebooks, Inc, Naperville, IL
1001 Healthy Baby Answers. Chapter: Food Allergy Page 262 – 267
Wasserman RL
Editors: .Sourcebooks, Inc, Naperville, IL
Abstracts/Posters
- Add-on omalizumab in children with uncontrolled moderate-severe allergic (IgE-mediated) asthma significantly reduces exacerbation rates.Milgrom H, Wasserman RL, Fowler-Taylor A, et al, 2009 ATS Annual Conference
- Oral Peanut Immunotherapy (OPI) in the Allergy Office.Mansfield LE, Wasserman R, Hutteman HR, Ruvalcaba M, Galluci A, ACAAI Annual Meeting 2009
- Patients Receiving C1-INH Treatment for Hereditary Angioedema Report Few Health-Related Limitations on Quality of Life Survey.Bewtra A, Levy R, Wasserman R, Jacobson K, Craig T, ACAAI Annual Meeting 2009
- Patient Data Capture System Reports Effectiveness of C1-INH Therapy for Acute Attacks of Hereditary Angioedema.Levy R, Bewtra A, Jacobson K, Wasserman R, Craig T, ACAAI Annual Meeting 2009
- Evaluation of Prodromal Symptoms Concurrent with Hereditary Angioedema Therapy.Prematta MJ, Craig T, Bewtra A, Levy R, Wasserman R, Jacobson K, Laudadio C, ACAAI Annual Meeting
- Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20, in patients with PID.Hagan JB, Fasano MB, Spector S, Wasserman RL, Melamed I, Rojavin MA, Zenker O, Orange JS, AAAAI 2010 International Conference
- Pharmacokinetics of IgPro20 after subcutaneous administration in patients with PID.Wasserman RL, Melamed I, Nelson RP, Knutsen AP, Fasano MB, Stein MR, Rojavin MA, Kiessling,P, Church J, AAAAI 2010 International Conference
- Pharmacokinetics and Safety of Subcutaneous Immune Globulin (Human), 10% Caprylate/Chromatography Purified (IGIV-C) in Patients With Primary Immunodeficiency Disease (…Wasserman RL, Irani AM,Gupta S, Tracy J, Tsoukas C, Stark D, Levy R, Roberts R, AAAAI 2010 International Conference
- Population Pharmacokinetics of C1 Esterase Inhibitor Used to Treat Patients with Acute Hereditary Angioedema Attacks in a Multicenter, Randomized, Placebo-controlled S…Ritchie B, Levy RJ, Wasserman RL, Bewtra AK, Hurewitz DS, Obtulowicz K, Reshef A, Moldovan D, Shirov T, Grivcheva-Panovska V, Bernstein JA, Kiessling PC, Schindel F, C…, AAAAI 2010 International Conference
- C1 Esterase Inhibitor: Retrospective Validation of a Commonly Used Endpoint in Hereditary Angioedema Studies, Time to Onset of Relief, in a Global, Multicenter, Random…Bernstein JA, Ritchie B, Levy RJ, Wasserman RL, Bewtra AK, Hurewitz DS, Obtulowicz K, Reshef A, Moldovan D, Shirov T, Grivcheva-Panovska V, Kiessling PC, Schindel F, C…, AAAAI 2010 International Conference
- IGIV 10%, Infused Intravenously and Subcutaneously to Subjects with Primary Immunodeficiency Diseases – Efficacy, Tolerability and Pharmacokinetic Comparison.Leibl H,Wasserman RL,Stein M, Melamed I, Schiff RI and the IGIV SC study group, First North American Primary Immune Deficiency Conference, 2010
- A prospective study (I.M.P.A.C.T.2) of rapid relief provided by C1 esterase inhibitor in emergency treatment of acute laryngeal attacks in hereditary angioedema.TJ Craig, Wasserman TL, Levy RJ, Bewtra AK, Schneider L, Packer F, Yang W, Keinecke HO, Kiessling PC, EAACI Annual Meeting, 2010
- Office Based Oral Desensitization of Patients With Anaphylactic Sensitivity to Foods Is Safe and Effective.Wasserman RL, Mansfield LE, Gallucci AR, Hutteman HR, Ruvalcaba AM, Long NA, Pence DM, AAAAI 2010 International Conference
- Office Based Oral Desensitization of Patients With Non-Anaphylactic Sensitivity to Foods Is Safe and Effective.Gallucci AR, Wasserman RL, Mansfield LE, Hutteman HR, Ruvalcaba AM, Long NA, Pence DM, AAAAI 2010 International Conference
- Tolerability of Human Immunoglobulin 10% Administered Subcutaneously Following Administration of Recombinant Human Hyaluronidase (rHuPH20) in Patients with Primary Imm…Gelmont D, Wasserman RL, Melamed I, Stein M, Rubinstein A, McCoy B, Engl W, Leibl H, Schiff RI, Grossman WJ, AAAAI 2011 International Conference
- Oral Immunotherapy (OIT) of Food Allergy (FA) Successfully Desensitizes Most Patients.Gallucci AR, Wasserman RL, Sugerman RW, Mireku-Akomeah N, Pence DM, Long NA, AAAAI 2011 International Conference
- Peanut Oral Immunotherapy (OIT) of Food Allergy (FA) Carries a Significant Risk of Eosinophilic Esophagitis (EoE).Wasserman RL, Sugerman RW, Mireku-Akomeah N, Gallucci AR, Pence DM, Long NA, AAAAI 2011 International Conference
- Subcutaneous Immunoglobulin Replacement Therapy for Primary Immunodeficiency: High-Dose versus Low-Dose Treatment using Hizentra.Hagan J, Wasserman RL, Zenker O, Borte M, AAAAI 2011 International Conference
- New Immune Globulin Subcutaneous (Human), 10% (IGSC) Product is Well Tolerated in Subjects with Primary Immunodeficiency Diseases (PIDD).Grossman WJ, Wasserman RL, Melamed I, Stein M, Leibl H, Schiff RI, Gelmont D, AAAAI 2011 International Conference
- Pharmacokinetic Analysis (PK) of Immune Globulin Subcutaneous (Human), 10% (IGSC) Administered Intravenously or Subcutaneously in Subjects with Primary Immunodeficienc…Leibl H, Wasserman RL, Melamed I, Stein M, Engl W,Schiff RI, Gelmont D, Grossman WJ, AAAAI 2011 International Conference
- Efficacy Analysis of Immune Globulin Subcutaneous (Human), 10% (IGSC) Administered Intravenously or Subcutaneously in Subjects with Primary Immunodeficiency Diseases (…Schiff RI, Wasserman RL, Melamed I, Stein M, Engl W, Leibl H,Gelmont D, AAAAI 2011 International Conference
- Pharmacokinetics (PK) of Human Immunoglobulin 10% Administered Subcutaneously Alone or Following Recombinant Human Hyaluronidase (rHuPH20) in Primary Immunodeficiency …Rubinstein A, Wasserman RL, Melamed I, Stein M, McCoy B, Engl W, Leibl H,Gelmont D, Schiff RI, AAAAI 2011 International Conference
- Efficacy Analysis of Immune Globulin Subcutaneous (Human), 10% (IGSC) Administered Intravenously or Subcutaneously in Subjects with Primary Immunodeficiency Diseases (…Schiff RI, Wasserman RL, Melamed I, Stein M, Engl W, Leibl H,Gelmont D, AAAAI 2011 International Conference
- Local Tolerability of Subcutaneous Infusions With 20% Immunoglobulin: Results From a Phase 3 Study in Subjects With Primary Immunodeficiency.Spector S, Wasserman RL, Melamed I, Rojavin MA, Hagan JB, Clinical Immunology Society Annual Meeting 2011
- Clinical Immunology Society Tolerability of Human Immunoglobulin 10% (IgG) Administered Subcutaneously (SC) or Facilitated With Recombinant Human Hyaluronidase (rHuPH2…Melamed I, Wasserman RL, Stein M, Rubinstein A, McCoy B, Engl W, Leibl H, Gelmont D, Schiff RI and the rHuPH20-facilitated IGSC Study Group, Clinical Immunology Society Annual Meeting 2011
- Rapid Clearance of L-Proline After Intravenous and Subcutaneous Infusion With L-Proline–Stabilized Immunoglobulin Replacement Products.Hagan JB, Robak T, Church JA, Rojavin M, Wasserman RL, Clinical Immunology Society Annual Meeting 2011
- Efficacy And Pharmacokinetics Of A New 10% Liquid Intravenous Immunoglobulin (Bivigam™ Ivig) In Patients With Primary Immunodeficiency.Wasserman RL, Church J, Strausbaugh S, Schroeder, Ballow M, Harris J, Melamed I, Elkayam D, Lumry W, Suez D, Rehman S. Safety, Clinical Immunology Society Annual Meeting 2011
- Tolerability and Efficacy of Recombinant Human Hyaluronidase (rHuPH20)-Facilitated Subcutaneous Infusion of Immune Globulin (Human), 10% (IGHy) in Patients with Primar…Stein M, Wasserman RL, Melamed I, Engl W, Leibl H, McCoy B, Gelmont D, Schiff RI, and the IGHy Study Group, ACAAI International Conference 2011
- Pharmacokinetics of Recombinant Human Hyaluronidase (rHuPH20)-Facilitated Subcutaneous Infusion of Immune Globulin (Human), 10% (IGHy) in Patients with Primary Immunod…Wasserman RL, Melamed I, Stein M, Engl W, Leibl H, McCoy B, Gelmont D, Schiff RI, and the IGHy Study Group, ACAAI International Conference 2011
- Safety and Pharmacokinetics of Facilitated-Subcutaneous Infusion of Immune Globulin (Human), 10% and Recombinant Human Hyaluronidase (IGHy) in a Phase III Extension St…Melamed I, Wasserman RL, Stein M, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI, and the IGSC, 10% rHuPH20 Study Group, AAAAI International Conference 2012
- Tolerability and Efficacy of Facilitated Subcutaneous Infusion of Immune Globulin (Human), 10% and Recombinant Human Hyaluronidase (IGHy) in a Subset of Study Patients…Wasserman RL, Melamed I, Stein M, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI, and the IGSC, 10% rHuPH20 Study Group, AAAAI International Conference 2012
- Pharmacokinetics (PK) of Human Immunoglobulin 10% (IG) Administered Intravenously (IGIV), Subcutaneously (IGSC) or Facilitated Subcutaneously with Recombinant Human Hy…Stein M, Wasserman RL, Melamed I, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI, and the IGSC, 10% rHuPH20 Study Group, AAAAI International Conference 2012
- Tolerability and Efficacy of Hizentra Over and Extended Period for the Treatment of Primary Immunodeficiency.Nelson RP, Malamed I, Stein MR, Wasserman RL, AAAAI International Conference 2012
- Cochlear Implantation in Adenosine Deaminase-deficient Severe Combined Immunodeficiency.Peters BR and Wasserman RL, 12th International Conference on Cochlear Implants and Other Implantable Auditory Technologies 2012
- Normalized Hearing and Improved Speech Discrimination Following Cochlear Implant in an ADA-SCID Patient.Wasserman RL and Peters BR, Clinical Immunology Society Annual Meeting 2012
- Tolerability and Efficacy of Facilitated-Subcutaneous Infusion of Human Immunoglobulin G, 10%, and Recombinant Human Hyaluronidase (IGHy): Subset of Study Patients Wit…Wasserman RL, Melamed I, Stein M, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI, and the IGSC, 10% rHuPH20 Study Group, European Society for Immunodeficiency Conference 2012
- Pharmacokinetics of Human Immunoglobulin G, 10%, Administered Intravenously (IGIV), Subcutaneously (IGSC) or Facilitated Subcutaneously With Recombinant Human Hyaluron…Stein M, Wasserman RL, Melamed I, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI and the IGSC, 10% rHuPH20 Study Group, European Society for Immunodeficiency Conference 2012
- Tolerability and Efficacy of Facilitated-Subcutaneous Infusion of Human Immunoglobulin G, 10%, and Recombinant Human Hyaluronidase (IGHy) in Patients With Primary Immu…Stein M, Wasserman RL, Melamed I, Rubinstein A, Puck J, Gupta S, Engl W, Leibl H, McCoy B, Gelmont D, Schiff I, and the rHuPH20-facilitated IGSC Study Group, European Society for Immunodeficiency Conference 2012
- Tolerability and Efficacy of Facilitated-Subcutaneous Infusion of Human Immunoglobulin G, 10%, and Recombinant Human Hyaluronidase (IGHy): Subset of Study Patients Wit…Wasserman RL, Melamed I, Stein M, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI, and the IGSC, 10% rHuPH20 Study Group, European Society for Immunodeficiency Conference 2012
- Long-Term Safety and Pharmacokinetics of Facilitated-Subcutaneous Infusion of Human Immunoglobulin G, 10%, and Recombinant Human Hyaluronidase (IGHy): Phase 3 Extensio…Melamed I, Wasserman RL, Stein M, Rubinstein A, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI and the IGSC, 10% rHuPH20 Study Group, European Society for Immunodeficiency Conference 2012
- Long-Term Safety and Pharmacokinetics of Facilitated-Subcutaneous Infusion of Human Immune Globulin G, 10%, and Recombinant Human Hyaluronidase: Phase 3 Extension Stud…Melamed I, Wasserman RL, Stein M, Rubinstein A, Puck J, Gupta S, Engl W, Leibl H, Gelmont D, Schiff RI and the IGSC, 10% rHuPH20 Study Group, ACAAI International Conference 2012
- Long-Term Efficacy and Tolerability of 20% SCIG in the Treatment of Patients With Primary Immunodeficiency Disease.Borte M, Wasserman RL, Rojavin MA, Bexon M, Jolles S, AAAAI International Conference 2013
- Characterization of the Effects of Proteolysis and Reduction on Cashew Allergens.Mattison CP, Grimm CC, Desormeaux WA, Wasserman RL, AAAAI International Conference 2013
- In vitro sensitization prior to fusion generates a high frequency of human-human hybridomas binding to group B streptococci (GBS).Wasserman RL, Kuhls TL, Pediatr. Res. 18:266A
- Primary in vitro immunization prior to fusion generates a high frequency of human-human hybridomas binding to azophenylarsonate.Wasserman RL, Budens R., Fed. Proc. 43:1680
- Human hybridoma antibodies specific for the group B streptococcal type III (GBS-III) polysaccharide.Wasserman RL, Pediatr. Res. 19:283A
- Human rheumatoid factors appear to use highly homologous variable region genes as a human anti-cytomegalovirus antibody.Newkirk MM, Ostberg L, Wasserman RL et al, Fed. Proc. 46:916
- Human monoclonal antibodies with specificity for the major allergen of mountain cedar pollen.Budens RD, Wasserman RL, Fed. Proc. 46:1001
- Human anti-hapten antibody diversity derives from combinatorial mechanisms employing few VH genes and several JH genes.Wasserman RL, Pediatr. Res. 21:319A
- Rheumatoid factor (RF) major cross-reactive idiotype expression in juvenile arthritis (JA) relates to VHI and VKIIIb gene utilization.Wasserman RL, Newkirk M, Fink CW, Pediatr. Res. 22:361A
- Rapid Infusion of a 12% concentration intravenous immunoglobulin (IVIG) product in children with Humoral Immunodeficiency.Hoffman M, Kobayashi RH, Wasserman RL, Kobayashi AD, Kiechel F, Sullivan M and Gelfand EW, AAAI. 1993 International Conference
- Immunologic Characterization of Allergic – Bipolaris- (Fungal) Sinusitis.Holman JM, Manning S, Gruchalla R, Wasserman RL, AAAI 1995 International Conference
- Albuterol Sulfate (AS) Interferes with the Ability of School Aged Children to Pay Attention.Wasserman RL and Steinfink DE, AAAI 1995 International Conference
- Sumatrypin is Effective for Treatment of Intravenous Gammaglobulin induced Headache.Wasserman RL, AAAAI 1996 International Conference
- Tumor Necrosis Factor Alpha (TNF) Induced by Intravenous Immunoglobulin (IVIG) Causes Hypotension In a Common Variable Immunodeficiency Patient (CVID).Wasserman RL, AAAAI 2001 International Conference
- Fluticasone Propionate is Safe in Treating Pre-School Age Children (24-47 Months) with Asthma.Wasserman RL, Kim KT, Blake K., et. al, AAAAI 2003 International Conference
- Efficacy, Safety and tolerability of a New Intravenous Immune Globulin, 10% Liquid, Triple Virus Reduced (IGIV, 10% TVR) in Patients With Primary Immunodeficiency (PID).Church JA, Leibl H, Stein MR, Melamed IR, Rubinstein A, Schneider LC, Wasserman RL, et. al, AAAAI 2005 International Conference
- Efficacy, A New Intravenous Immune Globulin, 10% Liquid, Triple Virus Reduced (IGIV, 10% TVR) – Pharmacokinetics, Efficacy and Safety in Patients with Primary Immunode…Church JA, Leibl H, Stein MR, Melamed IR, Rubinstein A, Schneider LC, Wasserman RL, et. al, 34th European Symposium on Clinical Pharmacy
- A Study to Evaluate the Handling Performance of Ventolin HFA MDI with Counter in Subjects with Asthma or COPD.Wasserman RL, Locantore NW, Carranza, et. al, AAAAI 2006 International Conference
- M.P.A.C.T. – The C1-Inh Program For HAE At Csl Behring. International, Multi-Center, Prospective, Angioedema, C1-Inh Trials.Craig T, Wasserman RL, Levy R, et. al, International HAE Patient Meeting, Frankfurt, Germany
- Use of Recombinant Human Hyaluronidase is Associated with Improved Bioavailability of Subcutaneously Administered Gammagard Liquid in Patients with Immunodeficiency Di…Schiff R, Wasserman RL, Stein M, et al, AAAAI 2008 International Conference
- Pharmacokinetics Of A Novel Liquid 10% Immunoglobulin Preparation For Intravenous Use (Privigen) In Subjects With Primary Immunodeficiency.Wasserman RL, Church Ja, Peter Hh, et al, AAAAI 2008 International Conference
- C1 esterase inhibitor (C1-INH) –Treatment of Acute Abdominal and Facial Attacks in Hereditary Angioedema (HAE): Results of the Global, Randomized Placebo controlled, P…Bernstein JA, Levy R, Wasserman RL, et al, AAAAI 2008 International Conference
- Effective And Safe Management Of Primary Immunodeficiencies With Privigen.Stein MR, Nelson RP, Church JA, Wasserman RL, et al, XIIIth Meeting of the European Society for Immunodeficiencies (ESID), Hertogenbosch, the Netherlands
- C1 esterase inhibitor (C1-INH) – Standard of Care for the Treatment of Acute Attacks in Hereditary Angioedema (HAE): Interim Results of an Ongoing, Prospective, Open L…Levy RL, Wasserman RL, Bewtra AB, et al, AAAAI 2009 International Conference
- Patient Driven Automated Data Collection System Demonstrates Effectiveness of C1INH Rescue Therapy.Levy RL, Bewtra AB, Craig TO, Hurewitz D, Wasserman RL, et. al, AAAAI 2009 International Conference
- An Office Based, One Day Rush Immunotherapy (1DRIT) Protocol for Pollens and Environmental Aeroallergens in Children.Wasserman RL, Sugerman RW, Marshall JD, Gallucci A, AAAAI 2009 International Conference
Other
- Common Infusion-Related Reactions to Subcutaneous Immunoglobulin Therapy: Managing Patient Expectations. Wasserman RL, Patient Preference and Adherence. 2008:2
- http://www.dovepress.com//common-infusion-related-reactions-to-subcutaneous-immunoglobulin-thera-pee